Metal Nanomaterials and Catalysis Research Group
(金属纳米材料及催化研究小组)
Materials with morphological features on the nanoscale usually have special properties stemming from their nanoscale dimensions (< 100 nm). Due to this reason, nanomaterials has the potential to play the major role in applications including catalysis, electronics, sensing, photonics, and nanomedicine, thus result in innovative technologies for improving the way we live.
Our research focuses on the rational design and synthesis of metal nanomaterials for different applications. This study will enable us to develop functional nanomaterials with optimized properties for fuel cells, catalytic converters, and water-splitting devices.
Research Interests
Project 1: Synthesis of noble metal nanocrystals with sub-10 nm dimension for applications in catalysis and electro-catalysis.
Over the past decades, noble metal nanocrystals have attracted considerable attention owning to their distinct properties and potential applications in catalysis, electronics, sensing, photonics, and nanomedicine. When used as a catalyst, the activity and/or selectivity of noble metal nanocrystals often have a strong dependence on their sizes and shapes. For example, the conversion efficiency and selectivity of alkynes to alkenes, conjugated alkenes to monoalkenes, and hydrogenation of alkenes are all strongly dependent on the size of Pd nanocrystals. On the other hand, it has been demonstrated that the facets on the surface of noble metal nanocrystals can have a strong influence on catalytic properties.
The last decade has witnessed the successful synthesis of noble-metal nanocrystals with a rich variety of shapes, with notable examples including cube, bar, cuboctahedron, octahedron, sphere, tetrahedron, right bipyramid, decahedron, icasahedron, plate, rod, and wire. However, most of these shapes can only be achieved in the size range larger than 10 nm, which are not suitable for real applications. As we know, noble metal nanocrystals used as heterogeneous catalysts usually exhibit a size smaller than 10 nm. That’s because noble metal nanocrystals with sub-10 nm dimensions will demonstrate different physical and chemical properties from large nanocrystals thus enable them with enhanced catalytic activities. By far, the challenge to synthetically and systematically tailor and fine-tune the shape of noble metal nanocrystals with sub-10 nm dimensions, and thus precisely control properties for practical catalysts, has met with limited success.
My research will focus on the shape-controlled synthesis of noble metal nanocrystals with sub-10 nm dimension and their related catalytic/electro-catalytic properties. The ultimate goal is to build a technology base for the large-scale production of functional noble metal nanostructures with optimized properties and sub-10 nm dimensions. So far, we have pioneered the synthesis of Pd nanocrystals in sub-10 nm dimension and evaluated their potential application toward CO oxidation reaction, see Figure 1. Based on the study on Pd nanocrystals, we will try to explore methodologies for the syntheses of other noble metal nanocrystals with sub-10 nm dimension. We believe this research will bridge up the gap between nanomaterials and practical catalysts, providing much optimized nano-catalysts for related reactions.

Figure 1. TEM images of Palladium nanocrystals enclosed by {100} facets with sizes around 6, 10, and 18 nm (top, from left to right). And the turnover frequencies of CO conversion at 140 ºC over ZnO-supported Pd cubes/bars with different sizes, show much enhanced catalytic activity of smaller Pd cubes/bars for low temperature CO oxidation reaction (bottom).
Project 2: Synthesis of transition metal nanocrystals and their applications in catalysis.
Controlling the morphologies of nanomaterials is one of the most important issues in nanotechnology. By far, remarkable progress has been achieved in the synthesis of noble metal NCs (Ag, Au, Pd, and Pt). In contrast, synthesis of transition metal nanocrystals is still in a rudimentary state relative to what has been achieved for noble metals.
Transition metal is of great importance in metallic material science because of their excellent catalytic, magnetic, optical and other properties. For example, Fe nanocrystals are very important catalysts in ammonia synthesis. Somorjai and co-workers have demonstrated that the surface structures of Fe catalysts have a significant impact on their reactivity. As for Ni, it was frequently used as an alloying constituent of catalysts and plays key role in the chemical and aerospace industries. Copper, another member of transition metal group, is also of great importance in metallic material science due to its high electrical nanostructures and excellent catalytic property. In methanol synthesis, Cu(110) facets of Cu NCs were reported to be more catalytically active than both Cu(100) and Cu(111) facets. Despite the wide applications of transition metals, the controlled synthesis of transition metal NCs have met with limited success by far. This difficulty can be attributed to the easiness of oxidation of transition metal NCs by the oxygen from air. One of the effective ways to address this challenge is to introduce stabilizers to avoid this oxidation during the synthesis. By using hexadecylamine as capping agent, we were able to generate Cu nanocrystals with different shapes including nanocubes and nanowires, see Figure 2.
This research will extend the shape-controlled synthesis of Cu to other transition metals, including Fe, Co, and Ni. The ultimate goal of this research is to synthesize transition metal nanocrystals with well-defined shapes in aqueous solution. Unlike the synthesis of noble metal nanocrytsals, the synthesis of transition metal was usually achieved in non-aqueous phase so that to avoid the oxidation of NCs during the formation process. Here we propose to address this oxidation issue in aqueous phase via the introduction of stabilizers which can avoid the oxidation of the as-prepared transition metal nanocrystals. Based on these transition metal nanocrystals with well-defined shapes, we will further uncover the structure-property relationships of these nanocrystals and catalytic reaction, aiming to find the most optimized structures for different catalytic reactions. This work will establish a new methodology for the fabrication of transition metal nanocrystals and bring some new transition metal nanostructures to a number of applications, like magnetism and catalysis.
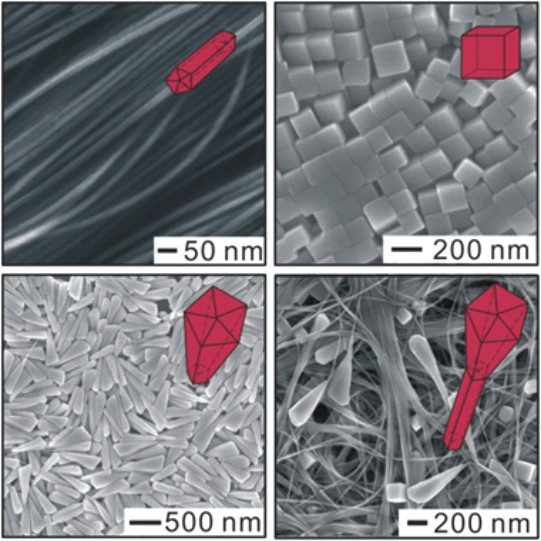
Figure 2. SEM images of Cu nanocrystals synthesized in aqueous solution in the presence of hexadecylamine.
Project 3: Construction of Bimetallic Nanocrystals for Applications in Fuel Cell.
Proton-exchange membrane fuel cells (PEMFCs), which efficiently convert the chemical energy of hydrogen into electricity with only water as a byproduct, possess great potential as substitutes for conventional combustion engines in future mobile applications owning to their high energy-efficiency and essentially no emission of pollutants. As the workhorse of many industrial catalysts, Pt is also a critical component of the PEMFC technology, where it acts as the most effective catalyst for the oxygen reduction reaction (ORR) and fuel (e.g., hydrogen) oxidation reaction. However, at present, PEMFCs are extremely expensive due to the precious metal component at the cathode. In addition, Pt cathode is vulnerable to the poisoning effect associated with the residual CO in a H2 stream during the hydrogen oxidation reaction. In order to decrease the cost and improve the efficiency of the Pt catalysts in PEMFCs, researchers have tried to alloy Pt with other metals, such as Fe, Co, Ni, and Cu. Generally, these alloys, which show much higher efficiency than Pt monometallic catalysts, were usually synthesized via the co-reduction reaction under a relative high temperature in non-aqueous solution. This synthetic process would call for large energy consumption thus result in high costs.
Recently, Xia and co-workers have found that Au-Ag alloy nanocages can be synthesized via the galvanic replacement reaction between Ag nanocubes and Au precursor in aqueous solution. In addition, my work on the synthesis of Pd-Pt alloy nanocages also showed the possibility to generate bimetallic alloy nanostructures via the galvanic replacement reaction (Figure 3).
My research will focus on the preparation of high efficient bimetallic Pt-M (M = Fe, Co, Ni, and Cu) catalysts for fuel cell applications. These nanocrystals can be generated via the galvanic replacement reaction between M(0) nanocrystals and Pt precursors in aqueous solution. The ultimate goal of this research is to synthesize transition metal nanocrystals with well-defined shapes in aqueous solution and use them as sacrificial templates for galvanic replacement reaction. Specifically, we can use Cu nanocrystals to prepare Pt-Cu bimetallic nanostructures and valuate the related electro-catalytic properties for fuel cell application. After the breakthrough is achieved for the synthesis of other transition metal nanocrystals, we can further extend this synthesis to other Pt-based nanostructures, such as Pt-Ni and Pt-Co bimetallic nanocrystals. The hollow structure as well as their alloy property can benefit for their applications in catalysis, thus would be important for material science.
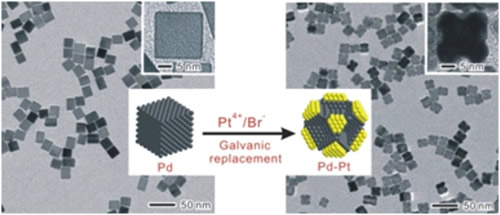
Figure 3. Synthesis of Pd-Pt Bimetallic Nanocrystals with a Concave Structure through a Bromide-Induced Galvanic Replacement Reaction.
Positions available:
Postdoctoral Research Associate: PhDs with research experience in chemical synthesis, material science, catalysis, and related areas are encouraged to apply. The salary will be in the range of 50,000-100,000 RMB (depending on experience) and an annual bonus up to 30,000 RMB is allocable (depending on performance). In addition, a 40-50 m2 apartment is provided during the contracted term.
Research Assistant: Maters with research experience in chemical synthesis, material science, catalysis, and related areas are encouraged to apply. The salary will be in the range of 30,000-40,000 RMB (depending on experience) and an annual bonus up to 20,000 RMB is allocable (depending on performance).
Graduate students: Students with Master or Bachelor degree of chemistry, physics, and materials science or a related discipline are all welcome to join my research group. If you are interested in our research, please contact to Mingshang Jin at mingshangjin@gmail.com.
Undergraduate students: Students majoring in chemistry, physics, and materials are also welcome to perform their Graduate Design and obtain experimental training in our research group.
