1). Thermodynamics of Porous Metal Oxide
Knowing the thermodynamic properties of metal oxide nanostructures is very important not only for the fundamental understanding of their chemical and physical properties but also for controlling their roles in a variety of industrial and environmental systems. During the past several decades, thermodynamic studies have been used to explore the stability of metal oxide nanocrystals with various sizes, shapes and polymorphs. Some touch upon the subject of thermochemistry, but none of them provide a comprehensive and rigorous summary of thermodynamic properties in a systematic fashion. By using high temperature calorimetry (Figure 1), we focus on (1) thermochemical data for the calculation of phase relations, of materials compatibility, and of optimal synthesis conditions and the relevance in providing insight into the factors relating to structure, bonding, stability, and reaction mechanisms. Questions to be addressed include: Are many different structures accessible, what is their energetic cost, and what microscopic features (for example, cation size, bond angles, bond lengths, covalency) favor or limit the formation of a given structure?

Figure 1. The setup of high temperature calorimetry
2). Nanoporous Metal for Green Fine Chemical Synthesis
Metallic foams are metals with pores deliberately integrated into their structures. It has attracted considerable attention due to their combinable properties that cannot be obtained with polymers, metals and ceramics. Various nanoporous metal including Au, Ag, Cu, Pt, Pd, Ni, Pt-Ru, Pt-Ni and Pt-Co have been synthesized and applied in the fields of electrocatalysis, surface enhanced Raman and sensors. There are several advantages for using nanoporous metal as catalysts for organic reactions: (1) Facile separation of catalysts from products. In contrast, the separation of metal colloids requires centrifugation,which is energy-consuming. (2) Robust structure of nanoporous metal. The metal colloids can aggregate due to the Ostwald repining and the size of the nanoparticles eventually becomes large, leading to less active catalysts for reactions. (3) Easy accessibility due to the clean interface between solute and catalysts. The surface of metal colloids is always capped by surfactants, which are charged and limit the accessibility of reactants to catalysts.
Another direction is the synthesis of nanoporous metal. To date, only a limited amount of nanoporous metals have been reported. Also, one may notice that studies focus on noble metals which are very expensive. Fabricating nanoporous metal catalysts based on less expensive metal is desired. Another focus should be bimetallic nanoporous catalysts with high activity and selectivity for organic reactions.
3). Metal/Porous Al2O3 Core/Shell Nanocatalysts for High Temperature Reaction
High temperature catalysis is important in many applications – biomass conversion, reforming reactions, and fuel cells to name a few. The metal nanoclusters anchored on porous substrates are commonly employed for high temperature catalysis. Generally, the catalysts should satisfy the following requirements: (1) Small nanoparticle catalysts are preferred. (2) Large surface area substrates are needed, simply because they can afford supporting a large number of small nanoparticles. (3) Catalysts should be stable at high temperatures. (4) The substrate itself needs to be rigid at high temperatures. Recently, a Pt/porous SiO2 core/shell nanoparticles proved to be extremely stable for CO oxidation. However, there are still two drawbacks for this system: (1) Catalysts are unstable at high temperatures (over 550 ° C) and (2) The thick porous SiO2 shell is unfavorable for diffusion of reactants and products. We propose to make nanocatalyst/porous Al2O3 core/shell nanostructures to preserve the large surface area of nanocatalysts and enhance stability of the catalysts that are active and stable at high temperatures under reactive conditions. The model system we will explore is Pt/porous Al2O3 core/shell nanoparticles for CO oxide reaction and water gas-shift reaction. In the following, we will explain works that will guide these research explorations.
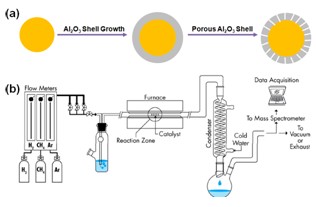
Figure 2. (a) Schematic of synthesis of Metal/Porous Al2O3 Core/Shell Nanocatalysts. (b) A designed reactor and detect system for water gas shift reaction.
