Bioactive Small Molecules Research GroupPrincipal Investigator: Dr. Pengfei Li All of our research programs are rooted in organic synthesis of small molecules. The drives of these programs are to discover bioactive small-molecule probes or drugs, to solve challenging synthetic problems, and/or to realize a theoretical hypothesis of general interests. Based on the traditional areas in organic chemistry, we conduct multidisciplinary researches either independently or in collaboration with groups of biology, medicine, chemical engineering, material science and computational science.
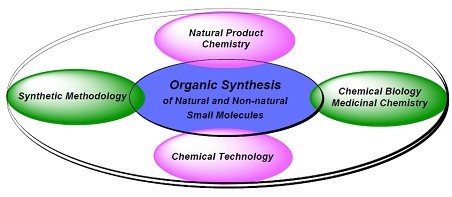
1. Total synthesis of natural products “Today, natural product total synthesis is associated with prudent and tasteful selection of challenging and preferably biologically important target molecules; the discovery and invention of new synthetic strategies and technologies; and explorations in chemical biology through molecular design and mechanistic studies. Future strides in the field are likely to be aided by advances in the isolation and characterization of novel molecular targets from nature, the availability of new reagents and synthetic methods, and information and automation technologies. Such advances are destined to bring the power of organic synthesis closer to, or even beyond, the boundaries defined by nature, which, at present, and despite our many advantages, still look so far away.” ---- The Art and Science of Total Synthesis at the Dawn of the Twenty-First Century K. C. Nicolaou et al[1]
[1] The Art and Science of Total Synthesis at the Dawn of the Twenty-First Century, K. C. Nicolaou, D. Vourloumis, N. Winssinger, P. S. Baran, Angew. Chem. Int. Ed. 2000, 39, 44-122.
Natural products have significantly contributed not only to the development of organic chemistry, but also to chemical biology and drug discovery. Total synthesis of complex natural molecules provide not only a platform for discoveries in organic chemistry, but also excellent training of students in understanding, design and practice of organic synthesis, which makes the students highly competitive in future research work. By studying the structure of a complex natural target and utilizing chemistry knowledge, design and implementation of a chemical synthesis of the molecule is a both logical and imaginative process. In our lab, we will develop efficient strategies and methods for synthesis of diverse natural products, especially those with important biological potentials. We are especially interested in unified strategies for synthesis of families of natural products and their analogs. The biological properties of these synthetic products will be studied in collaboration with other groups. A few representative synthetic targets are shown: 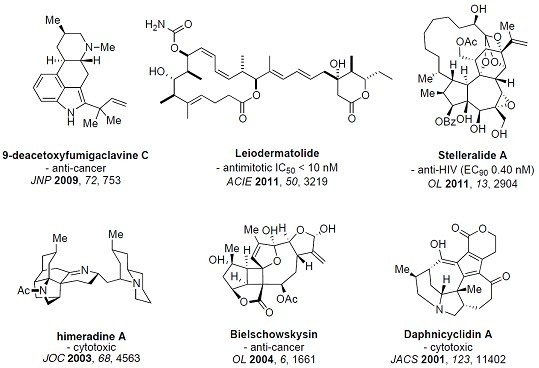
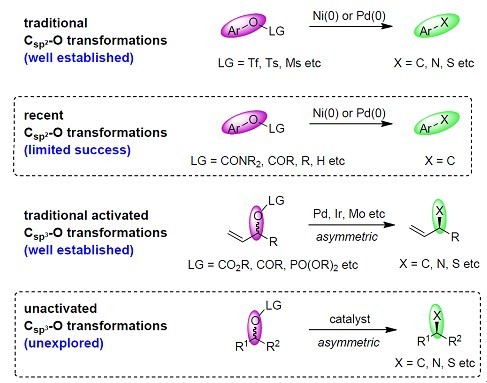
2. Development of synthetic methodology In this lab, novel and efficient synthetic methodology will be devised and developed either along total synthesis of natural products (natural-product-inspired and/or biomimetic methodology) or independently. The most interesting part will be catalytic stereoselective synthesis of molecular moieties that are present in many natural products or pharmaceuticals. One of the projects is transition-metal catalyzed C–O bond cleavage and transformations.[1],[2] Although Csp2–O bond cleavage has been a hot research topic in recent years and has seen certain success, more efficient and general methods are still needed. The largely unexplored unactivated Csp3–O bond transformations are especially interesting because of the wide presence of Csp3–O bonds in bulk chemicals and natural materials, and the possibility of stereoselective transformations to other functional groups.
[1] Selective, Nickel-Catalyzed Hydrogenolysis of Aryl Ethers, A. G. Sergeev, J. F. Hartwig, Science 2011, 332, 439-443.
[2] Exploration of New C–O Electrophiles in Cross-Coupling Reactions, D. -G. Yu, B. -J. Li, Z. J. Shi, Acc. Chem. Res. 2010, 43, 1486-1495.
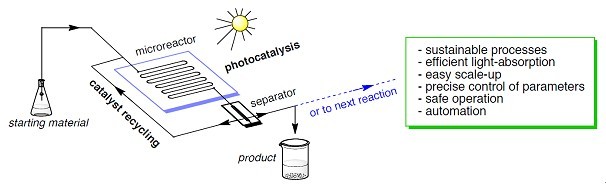
3. Continuous microflow chemistry Due to the higher ratio of surface area to volume, mass- and heat-transfer in microflow chemistry is more efficient than the one in a bulk mixture like in a flask. Therefore, more precise control of the reaction parameters is readily achievable. Furthermore, reaction intensification such as extreme reaction temperature, is possible in microflow chemistry. Consequently, to develop more efficient and more sustainable chemical processes, continuous microflow chemistry has been studied intensively now in several top institutes in the world. We are interested in developing otherwise difficult or ineffective chemical reactions using continuous microflow techniques. One project will be photocatalytic organic transformations under continuous microflow conditions.
4. Automated synthesis The ideal future chemistry should be done in highly automated systems using efficient and robust chemical methods. To produce libraries of new chemical entities (NCEs) in an efficient fashion in drug discovery, highly automated synthesis of complex molecules are required. Thus, robust and flexible chemistry must be developed. Polyketide natural products and their derivatives have proved important in medicinal chemistry. However, most syntheses of these molecules are lengthy, laborious and not suitable for large-scale production. In this lab, we are interested in developing modularly iterative strategies and automation systems toward synthesis of various polyketides. The automation systems may involve robots, microreactors and continuous-flow processes. 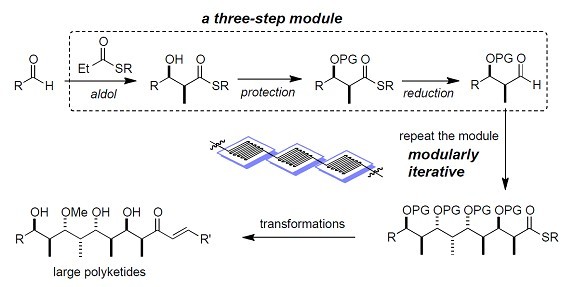
|